Draft ICH E9 addendum on Estimands and Sensitivity Analysis
20 November 2017 at Lundbeck A/S

Link to guideline: ICH E9 (R1) addendum on estimands and sensitivity analysis
Presentations from half-day meeting on Estimands – 26 Oct 2017

| Speaker | Presentation as pdf |
|---|---|
| Agenda | Detailed program |
| Frank Bretz | How the ICH E9 addendum around estimands may impact our clinical trials |
| Søren Andersen & Helle Lynggaard | Implementation of estimands in Novo Nordisk |
| Mette Krog Josiassen | Applying estimand strategies in schizophrenia |
Joint FMS/DSBS Meeting, Statistical analysis of risks and safety data
November 1, 2016, Radisson Blu Hotel, Malmö

The DSBS half-day seminar was held on 18 August 2016
Program and available presentations from the half-day seminar



Joint DSBS/DSTS Two-day Meeting 10-11 November 2015
Program and available presentations from the two-day meeting
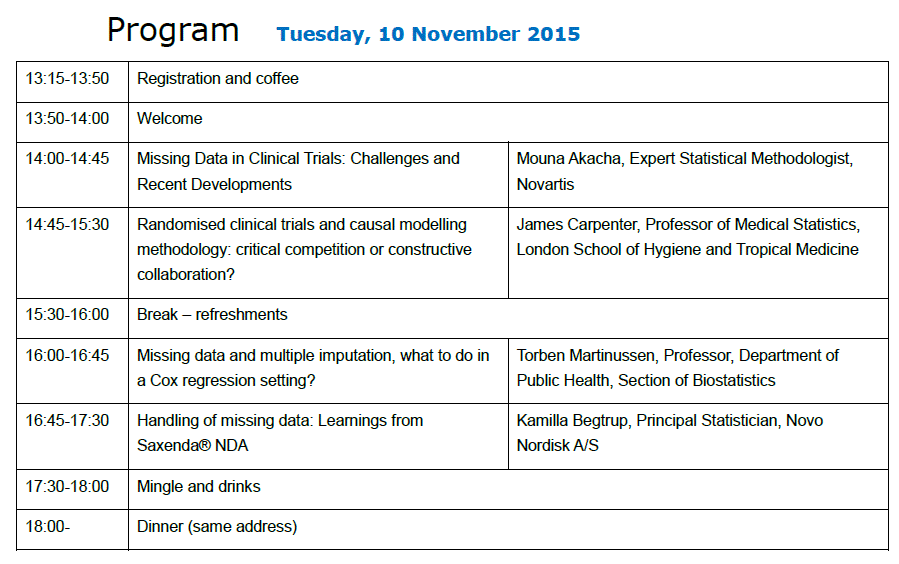
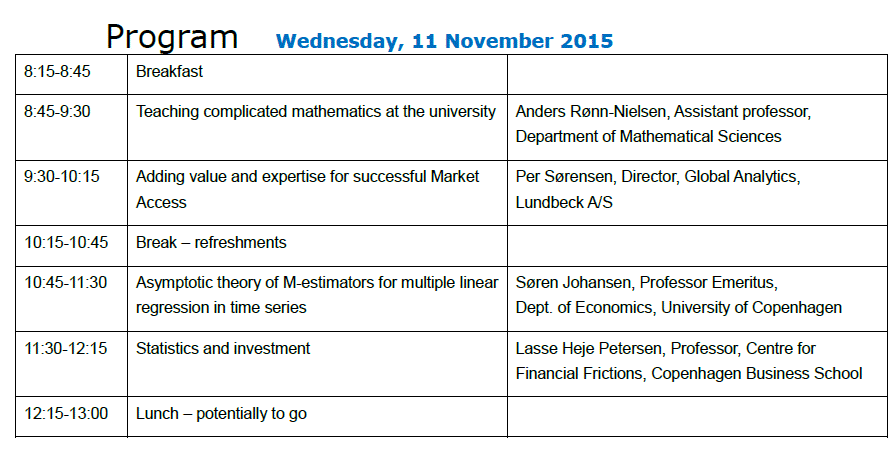





- Missing Data in Clinical Trials: Challenges and Recent Developments (Mouna Akacha)
- Randomised clinical trials and causal modelling methodology: critical competition or constructive collaboration? (James Carpenter) / Not yet available
- Missing data and multiple imputation, what to do in a Cox regression setting? (Torben Martinussen) / Available from the speaker upon request
- Handling of Missing Data: Learnings from Saxenda® NDA (Kamilla Begtrup)
- Teaching complicated mathematics at the university (Anders Rønn-Nielsen)
- Adding Value and Expertise for Succesful Market Access (Per Sørensen)
- A stochastic expansion of the Huber-skip estimator for multiple regression (Søren Johansen)
- Statistics and Investments: Efficiently Inefficient (Lasse Heje Pedersen)
Course material for Linear Mixed Models for Correlated and Repeated Measurements (March 2015)
- Introduction and longitudinal data analysis (Julie Lyng Forman)
- Introduction and longitudinal data analysis (4 slides per page)
- Linear mixed models (Julie Lyng Forman and Lene Theil Skovgaard)
- Linear mixed models (4 slides per page)
- Exercise: Bland Altman
- Exercise: CrossOver
- Exercise: NCGS cholesterol
- Exercise: Social Anxiety Disorder
- Exercise: TLC
- SAS program: Blandt Altman 1986 data.sas
- SAS program: Data for exercise Cholesterol NCGS.sas
- SAS program: Data for Exercise TLC.sas
Joint DSBS/FMS Meeting (October 2014)
- Open Data and Closed Minds? The pharmaceutical industry and its critics in the coming era of data-sharing (Stephen Senn)
- Mediation analysis – a tool to move from estimating treatment effect to understanding treatment mechanism (Theis Lange)
- Bayesian methods for the design of clinical trials in very rare diseases: application to the MYPAN trial in childhood polyarteritis nodosa (Lisa Hampson)
- A systematic application of good statistical practice in In-vivo studies (Janeli Sarv and Sofia Tapani)
- Using modern statistical methodology for validating and reporting Patient Reported Outcomes (Karl Bang Christensen)
- Evaluating dose-response under model uncertainty using several nested models (Corine Baayen)
- Challenges in design and analysis of large register-based epidemiological studies (Caroline Weibull and Anna Johansson)
DSBS meeting: Health Technology Assessment (May 2014)
- Example of Health Technology Assessment (HTA) of a therapy for the reduction of alcohol consumption (David Tyas)
- HTA-challenges in the Medical Device Industry (Jeppe Sørensen)
- EU HTA Assessment Outcome of Same Drug ? an Industry Perspective (Martin Strandberg-Larsen)
- It’s difficult to meet HTA criteria according AMNOG ? reason why ? (Oliver Macheleidt)
- Network meta-analysis in SAS (David A. Scott)
- Network Meta Analysis (NMA) Training (James Roger, Chrissie Fletcher and Neil Hawkins)
- SAS code for examples: smokingBinary.sas and diabetesContinuous.sas
Presentations at the annual general meeting (May 2014)
DSBS meeting: EMA draft guideline on the investigation of subgroups in confirmatory clinical trials (April 2014)
- Invitation
- Presentation of the EMA draft guideline on the investigation of subgroups in confirmatory clinical trials (Kristian Windfeld)
- EMA draft guideline on the investigation of subgroups in confirmatory clinical trials
- DSBS comments to the EMA draft guideline on the investigation of subgroups in confirmatory clinical trials
DSBS meeting: Statistical Issues in Drug development (October 2013)
- Invitation
- Responder endpoint and continuous endpoint, logistic regression or ANOVA? (Søren Andersen)
Presentations at the annual general meeting (May 2013)
DSBS meeting: The statistician in a regulated and global industry – Experiences and challenges (January 2013)
Benefit-Risk Assessment Methodology Workshop, Statistical Issues in Medical Statistics, FMS/DSBS 5th Joint Workshop (2012)
- Agenda and abstracts
- Benefit-Risk modelling of pharmaceuticals: Where are we now? (Larry Phillips)
- Quantitative Assessment of Benefit?Risk across the Product Lifecycle (Andrew Thomson)
- Comparison of Different Benefit-Risk Methods (Johan Bring)
- Data-driven assessment of the association of polymorphisms in 5-Fluorouracil metabolism genes with outcome in adjuvant treatment of colorectal cancer (Sinan B. Sarac)
- Post-marketing surveillance of drug safety in 2012: The EU Regulatory network (Doris Irene Stenver)
- Cost-benefit evaluations with applications in pricing & reimbursement of pharmaceuticals and in traffic safety (Ulf Persson)
- Benefit-Risk modelling of pharmaceuticals: Where are we going? (Larry Phillips)
Courses in Sample Size Determination in Clinical Trials and Meta analysis Using Individual Patient Data (January 2012)
Presentations at the annual general meeting (May 2011)
- The extended Williams? trend test – Background and practical example (Anders Malmberg)
- Exposure-Response Analysis ? Regulatory perspectives (Christoffer W. Tornøe)
DSBS meeting February 21, 2011
- Invitation
- Abstract on Practical aspects of large register studies: Multiple states and multiple timescales: The diabetes and cancer study (Bendix Carstensen)
- Practical aspects of large register studies: Multiple states and timescales (Bendix Carstensen)
- What is a biomarker and what can it be used for? (Philip Hougaard)
Presentations at the annual general meeting (May 2010)
- Discussion on Randomisation and Blinding (Lars Endahl)
- Perspectives on Non-Inferiority Clinical Trials ? based on draft FDA guidance doc (Kristian Windfeld)
Statistical Issues in Medical Statistics, 4th joint workshop (April 2010)
- Information
- Abstracts
- Future prospects for Medicon Valley (Peter Nordström)
- The Uppsala Biostatistical landscape in the 2000’s – an outsourcing perspective (Gary Jansson)
- Running a virtual department through outsourcing (Silvana Cappi)
- INSTRUMENT DEVELOPMENT – from an industry perspective (Anna Rydén)
- PK/PD Modelling using Stochastic Differential Equations (Henrik Madsen)
- Bayesian Statistics: are theoretical advances changing practice in the pharmaceutical industry and regulation of medicine? (Deborah Ashby)
Seminar on Regulatory Issues (May 2009)
- Sample size (Marc Andersen)
- Discussion on Multiple Testing (Lars Endahl)
- Missing data (Kristian Windfeld)
Estimating Sample Size in Clinical Trials (April 2009)
The role of the pharmaceutical statistician (March 2009)
The Analysis of Incomplete and Longitudinal Data (October 2008)
Introduction to PK modelling with stochastic differential equations in R (May 2008)
Statistical Issues in Medical Statistics, 3rd Joint Workshop (April 2008)
- Information
- Knowledge discovery in safety databases (Niklas Norén)
- Multivariate Analysis of Gene Expression Data a geometrical approach (Jens Nilsson)
- Risk Management Plan (Helge Gydesen)
- Alternative statistical modeling of Pharmacokinetics and Pharmacodynamics (Claus Dethlefsen)
- Deriving and interpreting PK parameters in a physiological model (Anders Källén)
Presentations at the annual general meeting (May 2007)
Group sequential and adaptive designs for clinical trials (May 2007)
Efficient design in phase II: Proof-of-concept studies and seamless progress to phase III (February 2007)
Seminar on Data Monitoring Commitee and safety (Annual general meeting, May 2006)
- Information
- Integrated Analyses of Safety – Data needed! (Marie Louise Valentin)
- DMC ? how and what should they evaluate? (Bjarne Nielsen)
- Analysis of Safety Data – Is More Enough? (Marc Andersen)
Statistical Issues in Medical Statistics, 2nd Joint Workshop (April 2006)
- Abstracts
- Dealing with censored data in linear and non-linear models (Wan Hui Ong Clausen og Birgitte Biilmann Rønn)
- Optimal design which are efficient for lack of fit tests (Frank Miller)
- Statistical validation of scales for measuring health related quality of life (Søren Lophaven)
- Statistical approaches to analyse interval-censored data in a confirmatory trial (Margareta Puu)
- Joint Modelling of Longitudinal Measurements and Survival Outcomes (Peter Diggle)
Clinical Trial Simulation (March 2006)
Multiple Testing: a clinical trial perspective (August 2005)
QT Prolongation (Annual general meeting, May 2005)
- Information
- Introduction to QT Prolongation Trials (Thomas Jon Jensen)
- Status on guidelines (Bjarne Nielsen)
